Our biological platform connects the various developments of the material-based platforms and investigates biological processes in an application-oriented manner. Interactions between materials and the biosystem are analyzed in vitro, in vivo and on a microbiological level. Focus areas of research are the quantitative assessment of cell-material and cell-cell interaction, co-culture systems of primary human cells, examination of the human immune response by biomaterials, and strategies for vascularization and 3D tissue perfusion.
Contact:
Dr. rer. nat. Tatjana Schilling
Telephone +49(0)931 201-73654
tatjana.schilling@uni-wuerzburg.de
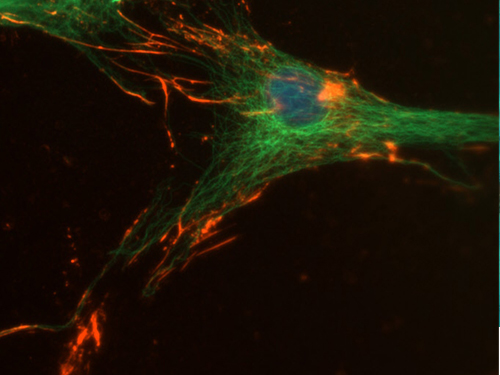
Double immunofluorescence on osteoblast like cells expressing osteopontin (red). Actin binding protein actinin is stained green.
Quantitative assessment of Cell-Material and Cell-Cell Interaction
Adhesion forces of cells onto biomaterials, as well as intercellular adhesion forces, are of major interest for different fields of biomaterial research and tissue evolution. Traditional approaches to quantitatively assess adhesion forces are limited to low forces and thus early timepoints of the adhesion, typically not more than a few minutes.
We employ a novel single cell technique called FluidFM®, where a conventional AFM cantilever is replaced with a hollow cantilever connected to a pumping system, which permits much higher forces to be exerted onto single cells. This setup allows to overcome the cell adhesion forces even after the complete maturation of the focal contacts, which are representative of the real culture conditions. We have advanced this approach and demonstrated that cells can even become detached out of confluent monolayers, which, when compared to the values for single adherent cells, give access to values of intercellular adhesion forces (Sancho et al. 2017)
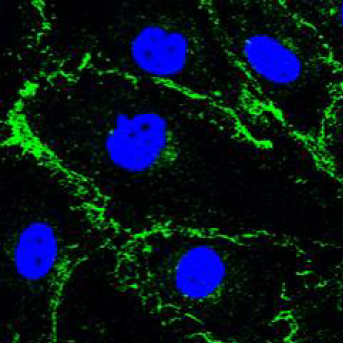
Human Umbilical Artery Endothelial cells (HUAEC). Adherens junction marker VE-Cadherin (green), nuclear marker TO-PRO-3 (blue)
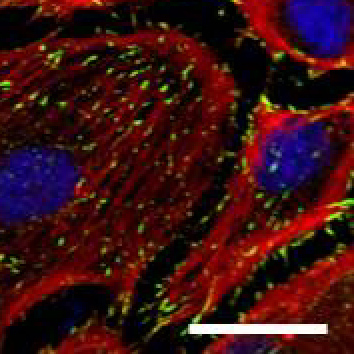
Human Umbilical Artery Endothelial cells (HUAEC). Focal adhesion marker vinculin (green), cytoskeletal marker phalloidin (red), nuclear marker TO-PRO-3 (blue), Scale bar 25µm
Co-culture systems of primary human cells
Traditionally, cytocompatibility of biomaterials is assessed using cell lines and single cell type experiments. For the evaluation of suitability of materials and scaffolds for certain applications, also primary cells are used. Accordingly, one area of activity in this platform is the initial cytocompatibility assessment of materials using standard cell lines, as well as more relevant functional assessment using more specific cell lines or also primary cells, such as human mesenchymal stromal cells. Those cells are imperative for regeneration of mesenchymal tissue and are employed in our lab to design novel biomaterials that promote cell function (i.e. differentiation processes).
In order to mimic cellular cross-talk and thus be closer to the in vivo situation, more complex culture assays, employing co-culture systems of cell lines, or eventually primary cells, are of major interest. Such systems are, however, non-standard and have to be established with regards to co-culture conditions in which all cell types maintain their phenotype and function. This is one main area of research within this platform. One example is the established co-cultivation of human macrophages and human mesenchymal stromal cells, which allows us to assess biomaterials in an in vivo related context of early stages after implantation.
Examination of the human immune response by biomaterial
The immune response to biomaterials is inevitable and thus a major criterium for designing and development of new biomaterials. Macrophages are core regulators of the host immune response to biomaterials. To prevent a prolonged inflammation, it is crucial to design biomaterials for tissue regeneration applications that attenuate the initial pro-inflammatory response and promote macrophage polarization into the regenerative type instead. Our focus is to gain knowledge about optimal biomaterial-design criteria and how those can enhance a regenerative outcome after implantation.
Furthermore, also neutrophils are one of the key regulators of the host immune response that is the first cell type interacting with the implant. The phagocytic activity is the primary function of neutrophils where they secrete antimicrobial substances and proteases, which are able to degrade ECM proteins (i.e. fibronectin, collagen IV). Here, we are interested to analyze the interaction of neutrophil enzymes with a polymeric biomaterial that is combined with diverse ECM components to promote cell functions (i.e. adhesion, differentiation).
Vascularization & 3D-Tissue Perfusion
One major challenge in the field of tissue engineering and biofabrication is the creation of biological constructs of relevant size. If this size exceeds the diffusion limit, living cells can not be supplied with nutrients and oxygen, leading to the creation of necrotic cores. Therefore, this platform also focuses on the investigation of possible vascularization and tissue perfusion strategies. The development is mainly based on melt electrowriting of thermoresponsive polymers, leading to the creation of perfusable, branched structures in the range of microvascularization.
Dr. rer. nat. Tatjana Schilling
Telephone +49(0)931 201-73654
tatjana.schilling@uni-wuerzburg.de
Dr. rer. nat. Kristina Andelovic
+49(0)931 201-73654
kristina.andelovic@uni-wuerzburg.de
Dr. rer. nat. Thorsten Keller
+49(0)931 201-73654
thorsten.keller@uni-wuerzburg.de
M. Sc. Vivien Priebe
+49(0)931 31-80587
vivien.priebe@uni-wuerzburg.de
EU Research Project DESIGN2HEAL Rational design of scaffold architecture and functionalization to induce healing and tissue regeneration
