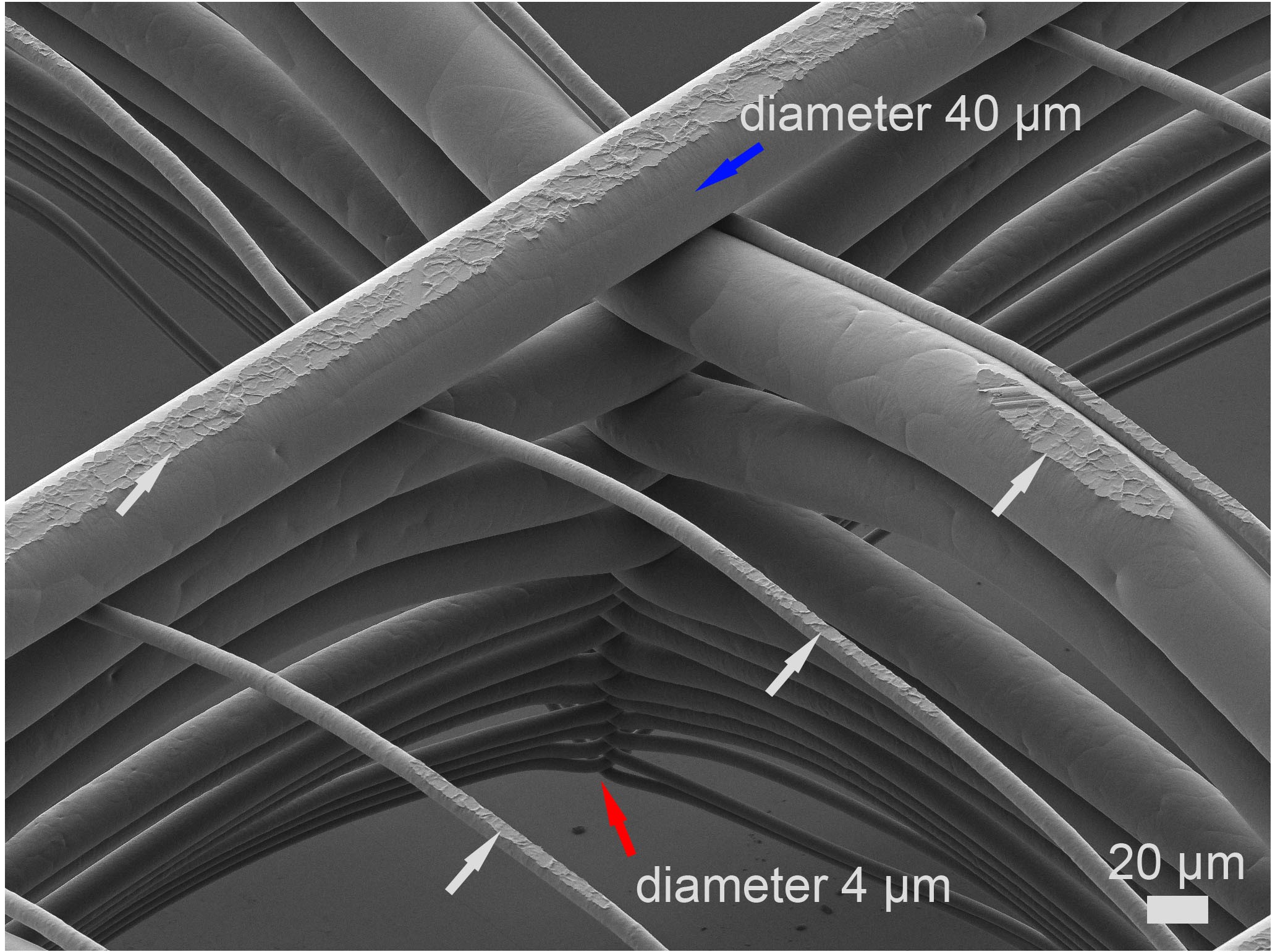
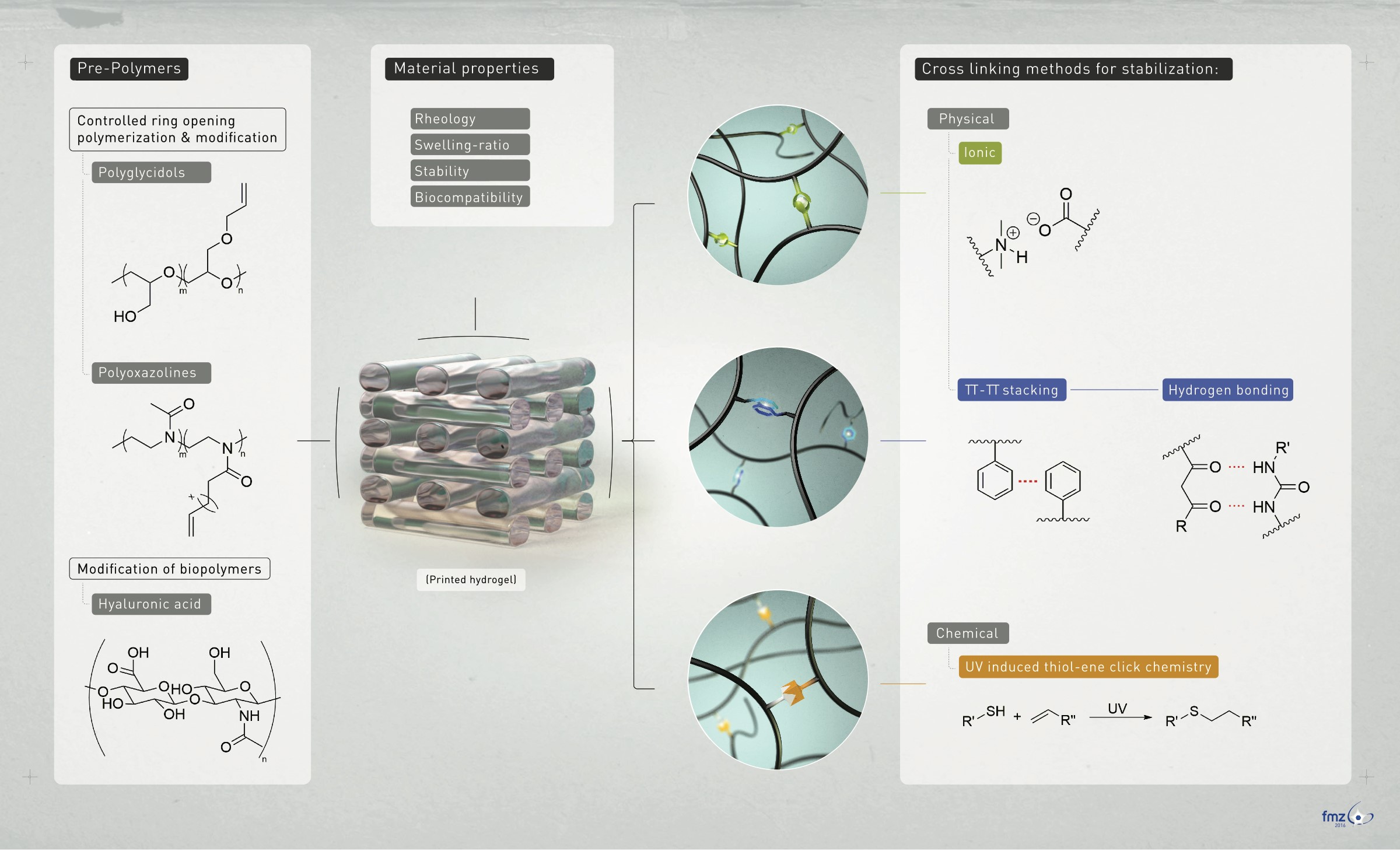
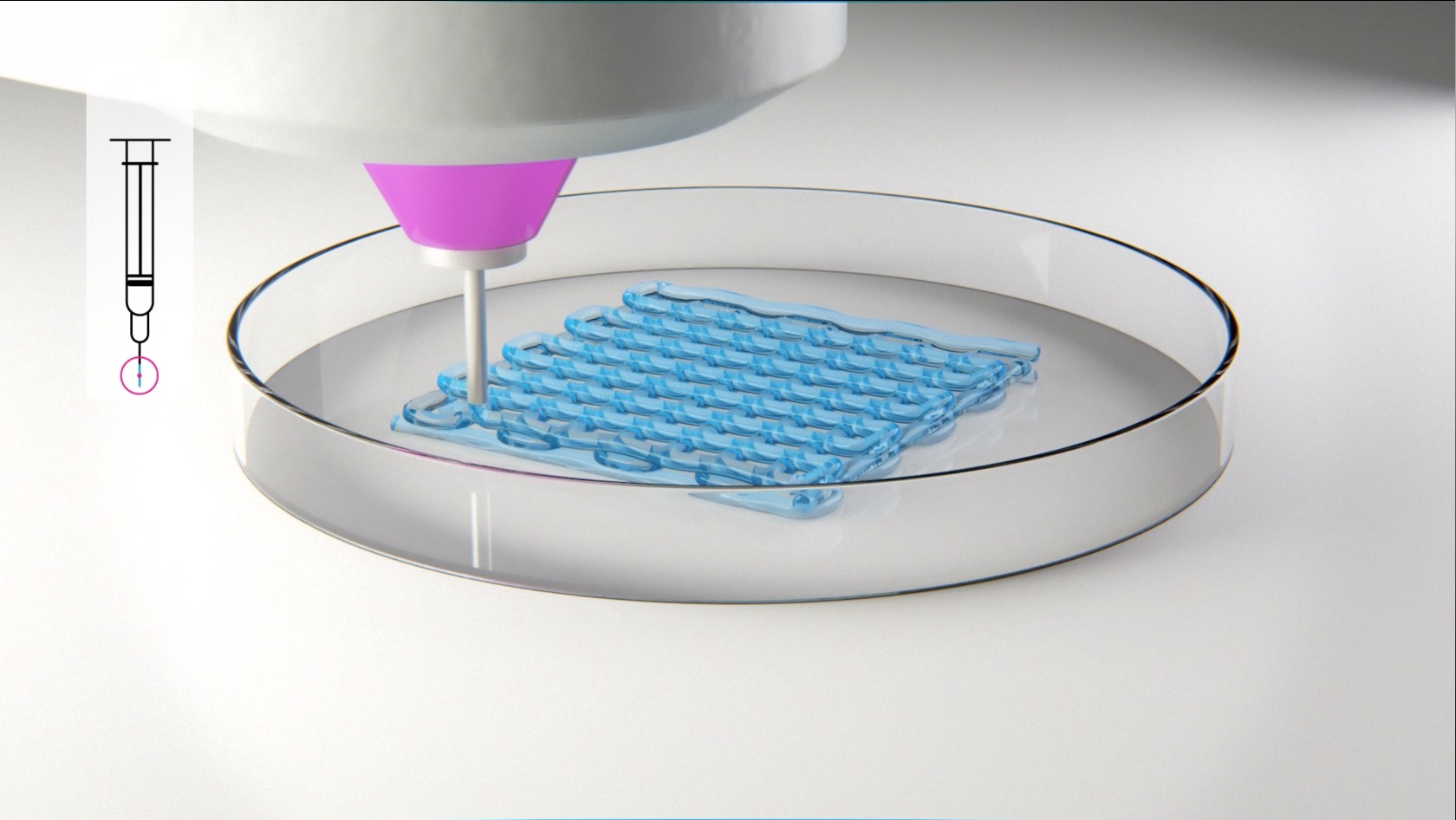
A bioink is defined as “a formulation of cells suitable for processing by an automated biofabrication technology that may also contain biologically active components and biomaterials” (Groll et al. 2019). The automated fabrication technology the FMZ is mainly utilizing is extrusion-based bioprinting. Bioink development at the FMZ (approach see figure 3) is focused on the combination of cells and biomaterials, more specifically the synthesis of hydrogel-based inks made from fully synthetic as well as modified natural polymers. According to the chosen polymers the applied synthetic techniques include cationic and anionic polymerizations of glycidols and polyoxazolines and subsequent polymer analogue sidechain modifications. For the natural polymers, different polysaccharides and proteins are used as starting materials to introduce further cross-linkable side functions. All synthesized and modified polymers are chemically characterized using the in-house available techniques (e.g. NMR, GPC, FT-IR, Raman, MALDI-TOF) to determine molecular weights, degree of modification and degradation or the branching geometry of synthesized materials.
After their synthesis the materials are investigated for their suitability as bioink components for the different below described techniques. This means that the hydrogel forming materials are crosslinked via radical polymerization, thiol-ene chemistry or reversible imine formation and then studied for their mechanical stability as well as swelling and degradation properties. Techniques applied for this are dynamic mechanical analysis, gravimetric evaluation as well as oscillation rheology.
Overall strategies for our bioink development are the investigation of new materials for bioinks, the optimization of existing materials using macromolecular chemistry and the development of materials for new printing approaches, as described below.
Contact:
Prof. Dr. Jürgen Groll
Telephone +49(0)931 201-73610
fmz-office@uni-wuerzburg.de
Dr. habil Jörg Tessmar
Telephone +49(0)931 201-73720
joerg.tessmar@uni-wuerzburg.de